Tailored Courses
Training designed around how your teams actually work
Off-the-shelf training often ticks boxes but doesn’t reflect the reality teams face day to day.
Our tailored training is designed for organisations that need learning to align with their trials, their teams, and their regulatory context — whether that’s inspection readiness, onboarding at scale, or responding to regulatory change.
Trusted by pharma, CRO, biotech, and academic research teams across Europe, the US and beyond.
We work closely with you to design training that fits:
your objectives and risk profile
your team roles and experience levels
your timelines, locations, and operational constraints
Areas we commonly tailor training around
While every programme is designed around your specific context, tailored training frequently covers areas such as:
Good Clinical Practice (GCP) and role-based GCP application
ISO standards and quality system implementation
Clinical Research Associate (CRA) onboarding and upskilling
Clinical Project Management (PM) for complex, multi-site studies
Audit and inspection readiness
Study or protocol-specific procedures
Delivery formats can include in-person workshops, live virtual sessions, blended programmes, or eLearning — always grounded in practical application, not theory alone.
Beyond individual topics
The examples above are just that — examples.
In practice, tailored programmes often combine multiple disciplines, roles, and learning formats, or are designed around a specific organisational challenge rather than a single topic.
This can include:
combining regulatory, quality, and operational perspectives
supporting cross-functional teams working on the same studies
aligning training with internal SOPs, systems, or ways of working
designing programmes that evolve over time as teams or trials progress
If you’re unsure whether what you have in mind fits a predefined category, that’s usually a good sign it’s worth discussing.
Who this is for
Tailored training is typically used by organisations that:
need consistency across teams or regions
are preparing for inspections or audits
are scaling teams without internal training capacity
want training that reflects how studies are actually run
If you’re an individual learner, our public courses may be a better fit — and we’re happy to help you find the right option.
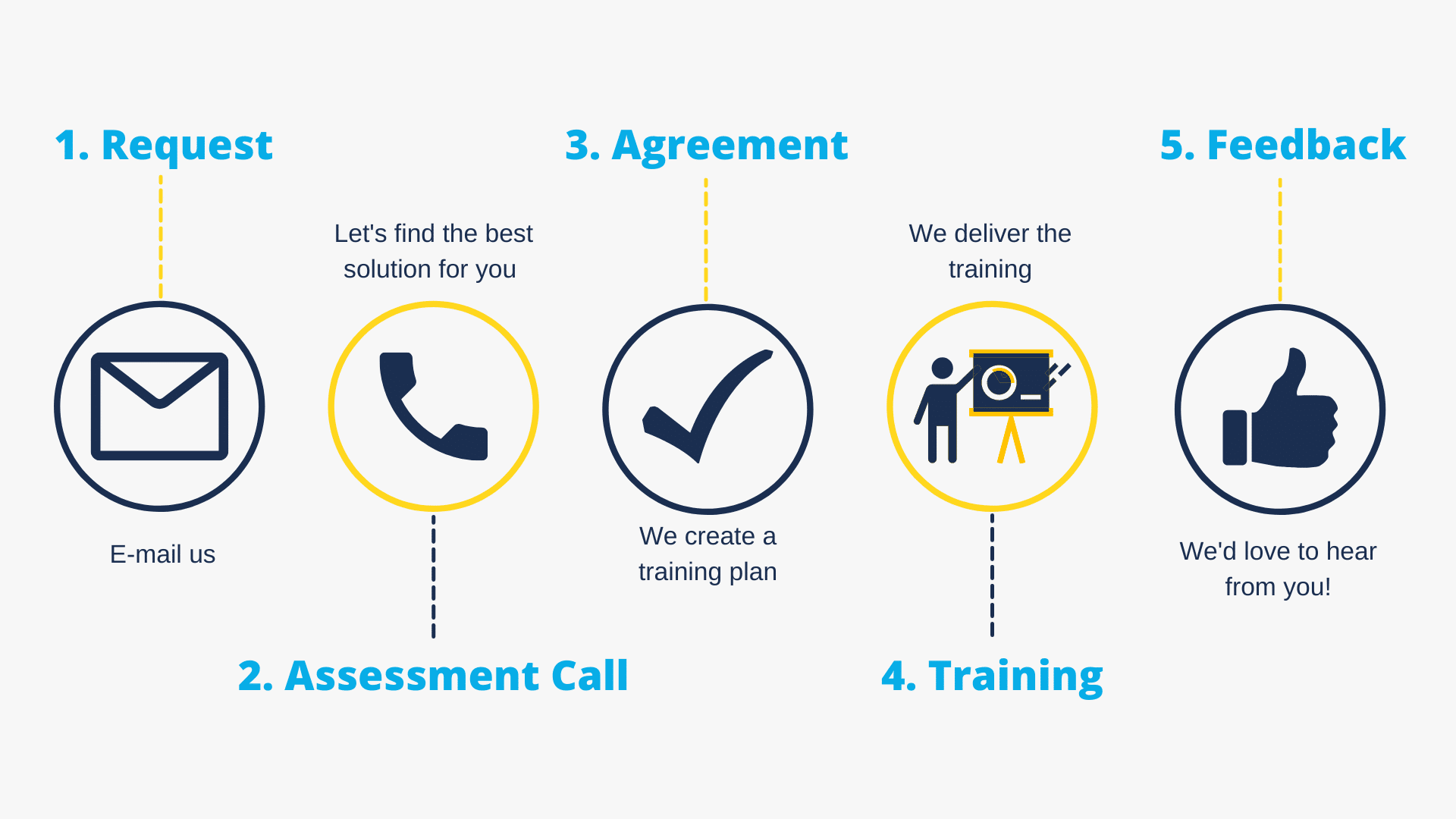
Let’s explore what makes sense for your teams
If you’re reviewing how training supports your teams, we’re happy to discuss your context and outline possible approaches — with no obligation.
Why ECCRT?

- Professional trainers with experience in the field and training ensure high-quality;
- Interactive training including case studies & workshops;
- Our extensive network of experts allow us to cover any training subject.
What trainings can we provide?

- Any type of clinical research related topics, such as regulatory, clinical operations and quality assurance courses;
- As well as soft-skills, that include communication, leadership and management courses;
- A combination of technical and soft skills can be chosen to ensure a holistic learning outcome;
- Our current courses can also be tailored upon your request.
Where can it take place?

- Anywhere in the world
- In your company or in another location of your choice;
- In our training facilities